Stanford scientists use nanotechnology to boost the performance of a key industrial catalyst
Nanoscale stretching or compressing significantly boosts the performance of ceria, a material widely used in catalytic converters and clean-energy technologies.
A tiny amount of squeezing or stretching can produce a big boost in catalytic performance, according to a new study led by scientists at Stanford University and SLAC National Accelerator Laboratory.

Chirranjeevi Gopal, former Stanford postdoctoral researcher, is lead author of the ceria study being published in “Nature Communications.” (Image credit: Courtesy Chirranjeevi Gopal)
The discovery, published May 18 in Nature Communications, focuses on an industrial catalyst known as cerium oxide, or ceria, a spongy material commonly used in catalytic converters, self-cleaning ovens and various green-energy applications, such as fuel cells and solar water splitters.
“Ceria stores and releases oxygen as needed, like a sponge,” said study co-author Will Chueh, an assistant professor of materials science and engineering at Stanford and a faculty scientist at SLAC. “We discovered that stretching and compressing ceria by a few percent dramatically increases its oxygen storage capacity. This finding overturns conventional wisdom about oxide materials and could lead to better catalysts.”
Catalytic converters
Ceria has long been used in catalytic converters to help remove air pollutants from vehicle exhaust systems.
“In your car, ceria grabs oxygen from poisonous nitrogen oxide, creating harmless nitrogen gas,” said study lead author Chirranjeevi Balaji Gopal, a former postdoctoral researcher at Stanford. “Ceria then releases the stored oxygen and uses it to convert lethal carbon monoxide into benign carbon dioxide.”
Studies have shown that squeezing and stretching ceria causes nanoscale changes that affect its ability to store oxygen.
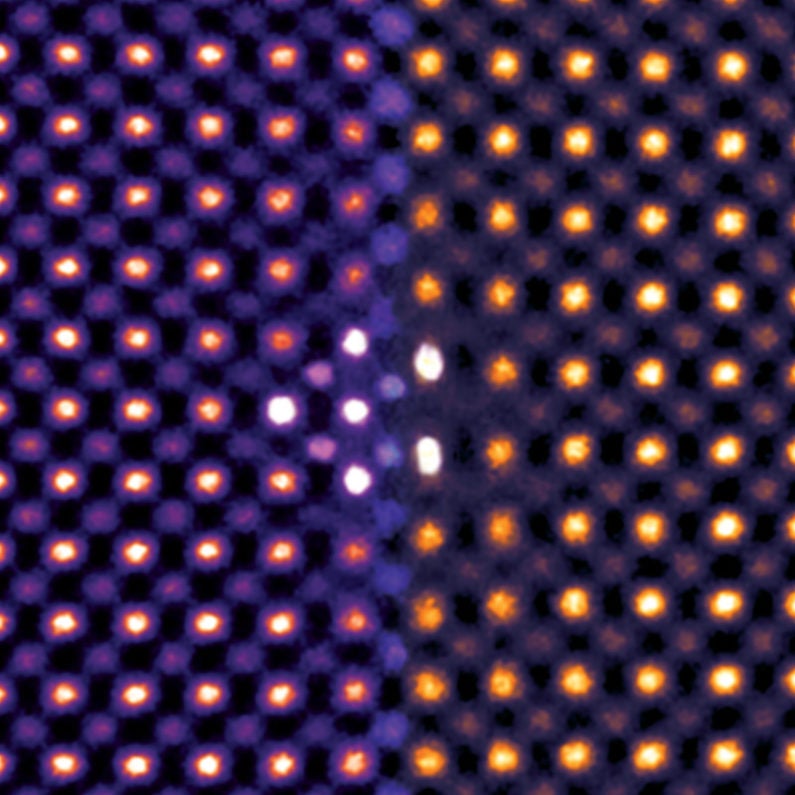
Colorized transmission electron microscopy of ceria ultrathin film reveals that individual atoms (shown as dots) shift under intense pressure. (Image credit: Sang Chul Lee)
“The oxygen storage capacity of ceria is critical to its effectiveness as a catalyst,” said study co-author Aleksandra Vojvodic, a former staff scientist at SLAC now at the University of Pennsylvania, who led the computational aspect of this work. “The theoretical expectation based on previous studies is that stretching ceria would increase its capacity to store oxygen, while compressing would lower its storage capacity.”
To test this prediction, the research team grew ultrathin films of ceria, each just a few nanometers thick, on top of substrates made of different materials. This process subjected the ceria to stress equal to 10,000 times the Earth’s atmosphere. This enormous stress caused the molecules of ceria to separate and squeeze together a distance of less than one nanometer.
Surprise results
Typically, materials like ceria relieve stress by forming defects in the film. But atomic-scale analysis revealed a surprise.
“Using high-resolution transmission electron microscopy to resolve the position of individual atoms, we showed that the films remain stretched or compressed without forming such defects, allowing the stress to remain in full force,” said Robert Sinclair, a professor of materials science and engineering at Stanford.
To measure the impact of stress under real-world operating conditions, the researchers analyzed the ceria samples using the brilliant beams of X-ray light produced at Lawrence Berkeley National Laboratory’s Advanced Light Source.
The results were even more surprising.
“We discovered that the strained films exhibited a fourfold increase in the oxygen storage capacity of ceria,” Gopal said. “It doesn’t matter if you stretch it or compress it. You get a remarkably similar increase.”
The high-stress technique used by the research team is readily achievable through nanoengineering, Chueh added.
“This discovery has significant implications on how to nanoengineer oxide materials to improve catalytic efficiency for energy conversion and storage,” he said. “It’s important for developing solid oxide fuel cells and other green-energy technologies, including new ways to make clean fuels from carbon dioxide or water.”
Other Stanford co-authors of the study are Max Garcia-Melchor, now at Trinity College Dublin (Ireland), and graduate students Sang Chul Lee, Zixuan Guan, Yezhou Shi and Matteo Monti. Additional co-authors are Andrey Shavorskiy of Lund University (Sweden) and Hendrik Bluhm of Lawrence Berkeley National Laboratory.
This work was supported by the U.S. Department of Energy, the National Science Foundation and the Stanford Precourt Institute for Energy.
