Stanford scientists develop new method to faster – and more accurately – find antigens that trigger specific immune cells
Their approach, which mimics the physical forces exerted by immune cells as they crawl over host cells, could help scientists develop more effective cancer immunotherapies.
A cell’s secrets can be divulged by its surface, decorated with tens to hundreds of thousands of molecules that help immune cells determine friend from foe. Some of those protruding molecules are antigens that trigger the immune system to attack, but it can be difficult for scientists to identify those antigens, which often vary across individuals, in the molecular forest.
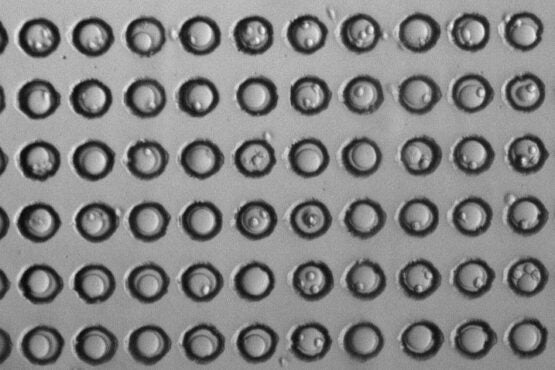
The microwells in each experiment, seen in this micrograph by the black outlines, were loaded with spherical beads (the large grey circle in each well), and T cells (the smaller circles adhering to some of the beads). (Image credit: Yinnian Feng)
A team of Stanford scientists led by Polly Fordyce, an Institute Scholar at Sarafan ChEM-H, has developed a new method to faster and more accurately predict which antigens will lead to a strong immune response. Their approach, which was reported in Nature Methods on Sept. 5, could help scientists develop more effective cancer immunotherapies.
T cells, a class of immune cells, crawl along and squish past other cells as they patrol the body, using T cell receptors to molecularly read peptides, or short pieces of proteins – which are cradled within larger proteins called major histocompatibility complexes (pMHCs) that project from cell surfaces. Healthy host cells display an array of pMHCs that do not trigger an immune response, but once T cells recognize disease-indicating peptides, they become activated to find and kill cells bearing these foreign signatures. Understanding how T cells sensitively distinguish these antigenic peptides from host peptides to avoid mistakenly killing host cells has long been a mystery.
“A T cell can detect a single antigenic peptide amongst a sea of 10,000 or 100,000 non-antigenic peptides being displayed on cell surfaces,” said Fordyce, assistant professor of bioengineering and of genetics.
The key to selectivity is in the T cell crawl. T cells’ sliding puts stress on the bonds between receptors and peptides, and in most cases, that extra stress is enough to break that bond. But sometimes, it has the opposite effect. Chris Garcia, co-author of the study and professor of molecular and cellular physiology and of structural biology, and others had already shown that the most antigenic peptides are those whose interactions with T cell receptors grow stronger in response to sliding.
“It’s kind of like a Chinese finger trap,” said Fordyce. “When you pull a bit at the receptor-antigen interaction, the binding actually lasts longer.”
Cellular mimicry
Identifying the best antigen-receptor pairs requires simultaneously applying that sliding, or shear, force between a peptide and a T cell and measuring T cell activation, ideally thousands of times to get repeatable data for many possible peptide/T cell receptor pairs. But existing methods are time-intensive and can result in measuring only one peptide with hundreds of T cells in a day.
The study’s first author, postdoctoral scholar Yinnian Feng, developed a trick that allows the team to measure 20 unique peptides interacting with thousands of T cells in less than five hours.
To make a simplified system that mimics cells with dangling peptides, they constructed small spherical beads from a material that expands upon heating and attached a few molecules of a given peptide-studded pMHC to their surfaces. After depositing a T cell atop each bead and waiting long enough for receptors to bind to the peptides, they then very slightly heated the bead. The bead’s expansion increases the distance between tether points, and the corresponding stretching of the T cell mimics the force it would experience sliding along cells in the body. After exerting that force, the team then measured how active the T cells were.
They could do hundreds of individual experiments in parallel by using beads that are each labeled with a unique color, making it possible to track multiple different pMHCs. They took two sets of pictures tiling across each slide after each run: one set that tells them which pMHC a given bead is displaying and another that tells them how active each T cell atop that bead is. Cross-referencing those images tells them which antigens led to the strongest T cell responses.
In this demonstration of their platform, the team showed, with 21 unique peptides, that their results confirmed known activating and non-activating peptides for one T cell receptor and uncovered a previously unknown antigen that induced a strong T cell response. Working with the Garcia lab, they have also already begun to address a challenge in immunotherapy: the T cell receptors that form the highest affinity interactions with antigens in the lab are often also activated by non-antigenic peptides in the body, a dangerous side effect that leads to the killing of healthy cells. Using their technology, the team characterized T cell receptors engineered to specifically recognize tumor antigens without off-target reactivity. In future work, they plan to build libraries of over 1,000 peptides to uncover novel antigens.
They hope that this approach, which is quick and requires few cells, or an optimized form of it could one day be used to improve personalized immunotherapies.
“This platform can help improve efforts to engineer T cells that specifically target cancer cells, as well as determine which antigens are capable of potently activating a patient’s own T cells to more effectively target cancer cells,” said Fordyce.
Fordyce is a member of Stanford Bio-X, SPARK, and the Wu Tsai Neurosciences Institute, and is a Chan Zuckerberg Biohub investigator. Garcia is a member of Stanford Bio-X, the Stanford Cancer Institute, the Wu Tsai Neurosciences Institute, and a Howard Hughes Medical Institute investigator.
Xiang Zhao and Adam K. White are also authors of the paper.
The work was funded by a Stanford Bio-X Interdisciplinary Initiatives seed grant and the National Institutes of Health.
