Whether charging a phone or powering the TV remote, most people are well-acquainted with batteries. But diving deeper into the science of batteries reveals a wealth of surprising ideas and innovations. Although they’ve been a familiar technology for decades, batteries are set to be an important technology of the future.
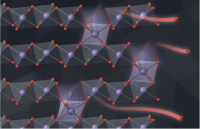
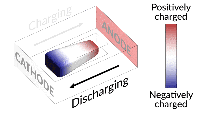
Inside all batteries are electrochemical cells that store chemical energy with the potential to be converted into electrical energy. Most batteries have a positively charged side (the anode) and a negatively charged side (the cathode). When the electrons flow from the anode to the cathode through a circuit, the battery can power other electrical elements added to the system, like lightbulbs. This simple structure opens up opportunities for probing and fine-tuning across many disciplines, and Stanford University researchers are doing just that.
As they work to solve the mysteries of battery degradation, reveal the true environmental toll of battery production and disposal, and improve the performance of next-generation batteries, battery researchers are hoping their advances can change the world – and our daily lives – for the better.