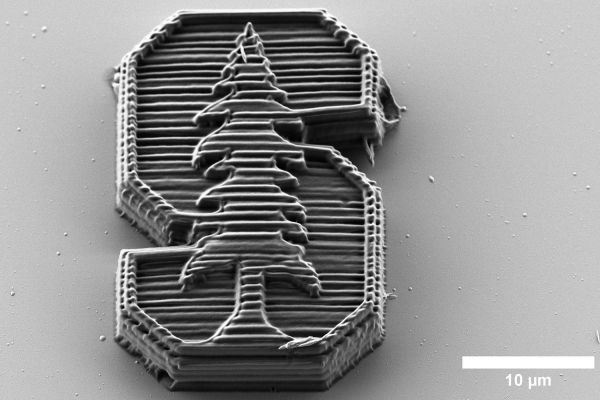
Science fiction envisions rapid 3D printing processes that can quickly create new objects out of any number of materials. But in reality, 3D printing is still limited in the properties and types of materials that are available for use, especially when printing at very small scales.
Researchers at Stanford have developed a new material for printing at the nanoscale – creating structures that are a fraction of the width of a human hair – and used it to print minuscule lattices that are both strong and light. In a paper published in Science, the researchers demonstrated that the new material is able to absorb twice as much energy than other 3D-printed materials of a comparable density. In the future, their invention could be used to create better lightweight protection for fragile pieces of satellites, drones, and microelectronics.
“There’s a lot of interest right now in designing different types of 3D structures for mechanical performance,” says Wendy Gu, an assistant professor of mechanical engineering and a corresponding author on the paper. “What we’ve done on top of that is develop a material that is really good at resisting forces, so it’s not just the 3D structure, but also the material that provides very good protection.”
Introducing metal nanoclusters
To design a better material for 3D printing, Gu and her colleagues incorporated metal nanoclusters – tiny clumps of atoms – into their printing medium. The researchers are printing with a method known as two-photon lithography, where the printing material is hardened through a chemical reaction initiated by laser light. They found that their nanoclusters were very good at jump-starting this reaction and resulted in a material that was a composite of the polymer printing medium and metal.
“The nanoclusters have very good properties for taking in the laser light, and then converting that to a chemical reaction,” Gu says. “And they’re able to do this with several classes of polymers, so they’re even more versatile than I expected.”
The researchers were able to combine metal nanoclusters with acrylates, epoxies, and proteins – several common classes of polymers that are used in 3D printing. Moreover, the nanoclusters helped to speed up the printing process. By combining the nanoclusters with proteins, for example, Gu and her colleagues were able to print at a rate of 100 millimeters per second, which is about 100 times faster than had previously been achieved in nanoscale protein printing.
The researchers tested their new material with several different lattice structures, prioritizing the ability to carry a heavy load in some and the ability to absorb an impact in others. With the nanocluster-polymer composite, all the structures demonstrated an impressive combination of energy absorption, strength, and recoverability – essentially the ability to squish and spring back.
“The lattice structure certainly matters, but what we’re showing here is that if the material it’s made out of is optimized, that’s more important for performance,” Gu says. “You don’t have to worry about exactly what the 3D structure is if you have the right materials to print with.”
Copying the natural world
In some ways, Gu and her colleagues are trying to mimic what nature has already perfected. Bone, for example, gets its resilience from the combination of a hard exterior, nanoscale porosity, and small amounts of soft material. This combination of a 3D structure and multiple, well-designed materials allows our bones to transfer energy without breaking (most of the time) and still remain relatively lightweight. Ideally, 3D-printed protective structures would also have multiple types of material within them, some harder and some softer, to better disperse an impact and resist crushing.
“Since the nanoclusters are able to polymerize these different classes of chemicals, we may be able to use them to print multiple materials in one structure,” Gu says. “That’s one thing we’d like to aim for.”
Additional Stanford co-authors of this research include postdoctoral scholars Qi Li and Ottman A. Tertuliano; and graduate students John Kulikowski, David Doan, and Melody M. Wang. Other coauthors are from Northwestern University. Gu is also a member of Stanford Bio-X.
This work was funded by the National Science Foundation, the American Chemical Society Petroleum Research Fund, and a Stanford Graduate Fellowship.
To read all stories about Stanford science, subscribe to the biweekly Stanford Science Digest.
Media Contacts
Jill Wu, Stanford University School of Engineering: (386) 383-6061; jillwu@stanford.edu