Using a series of more than 1,000 X-ray snapshots of the shapeshifting of enzymes in action, researchers at Stanford University have illuminated one of the great mysteries of life – how enzymes are able speed up life-sustaining biochemical reactions so dramatically. Their findings could impact fields ranging from basic science to drug discovery, and provoke a rethinking of how science is taught in the classroom.
“When I say enzymes speed up reactions, I mean as in a trillion-trillion times faster for some reactions,” noted senior author of the study, Dan Herschlag, professor of biochemistry in the School of Medicine. “Enzymes are really remarkable little machines, but our understanding of exactly how they work has been lacking.”
There are lots of ideas and theories that make sense, Herschlag said, but biochemists have not been able to translate those ideas into a specific understanding of the chemical and physical interactions responsible for enzyme’s enormous reaction rates. As a result, biochemists don’t have a basic understanding and, therefore, have been unable to predict enzyme rates or design new enzymes as well as nature does, an ability that would be impactful across industry and medicine.
“Using these detailed ensembles of enzyme states, we’ve been able to quantify and rigorously explain in chemical detail what features in enzymes provide catalysis and by how much,” said the study’s first author, Siyuan Du, a doctoral student in Herschlag’s lab. Their study appears in the Feb. 13 issue of the journal Science.
“Our new approach – and understanding – starts us down a road to be able to design enzymes that rival those found in nature, though this is just the start, and much more work is needed to achieve that goal,” Herschlag added.
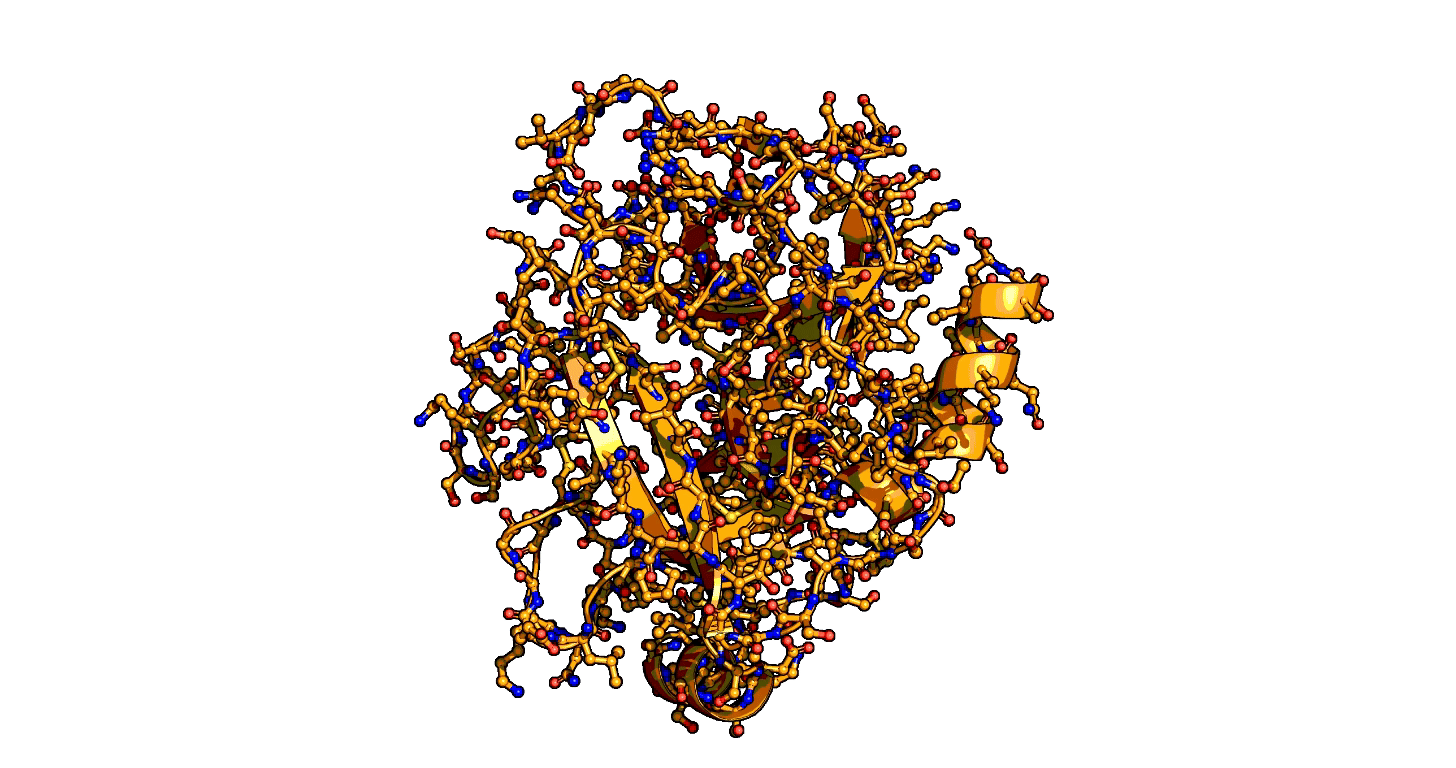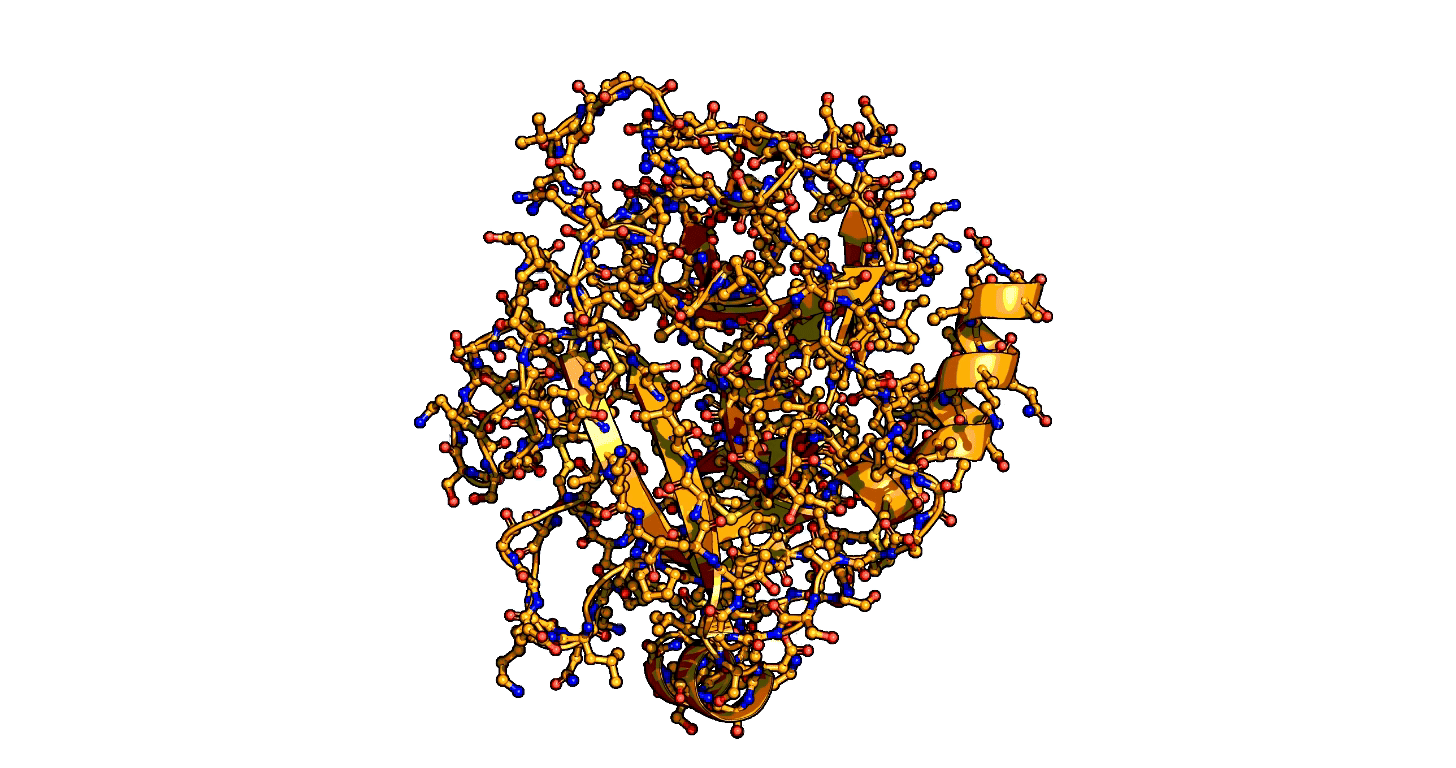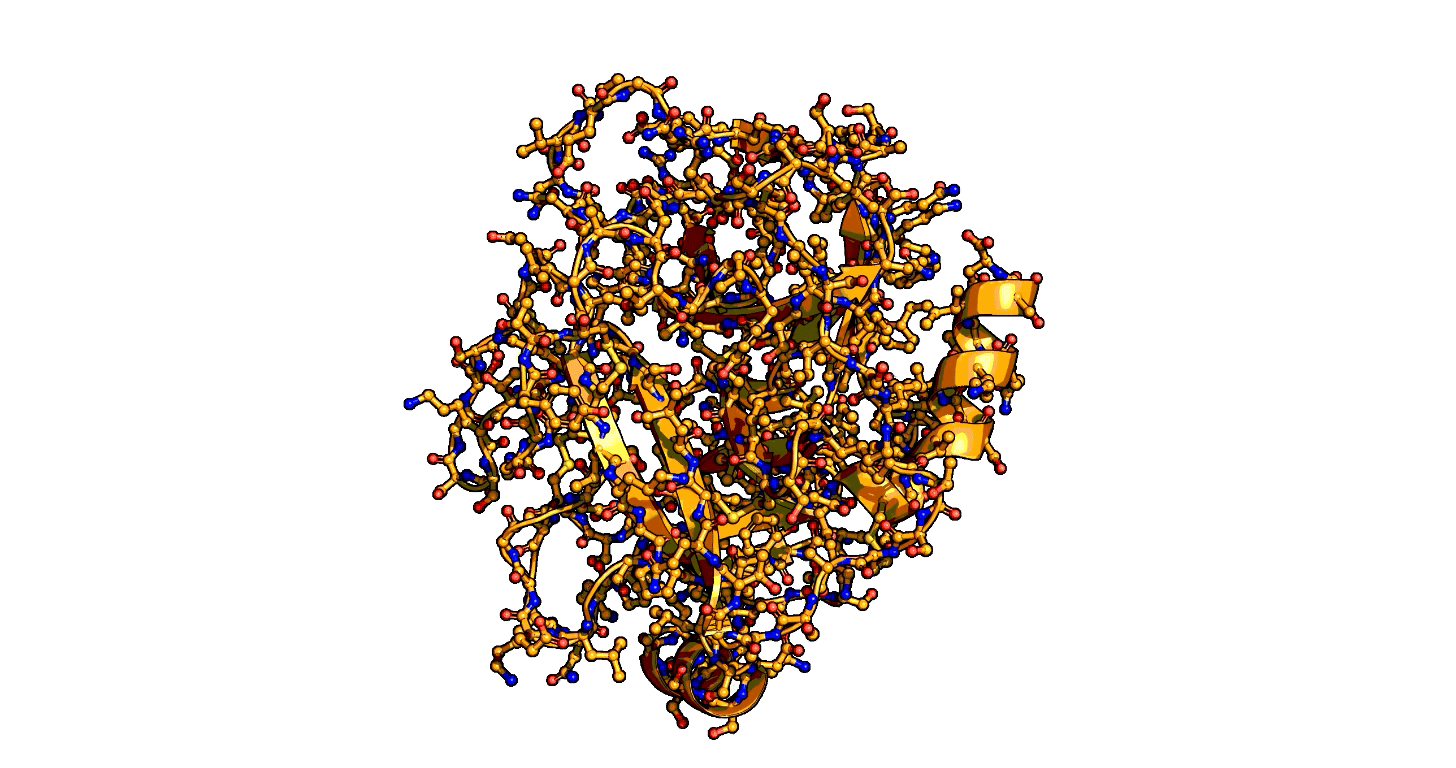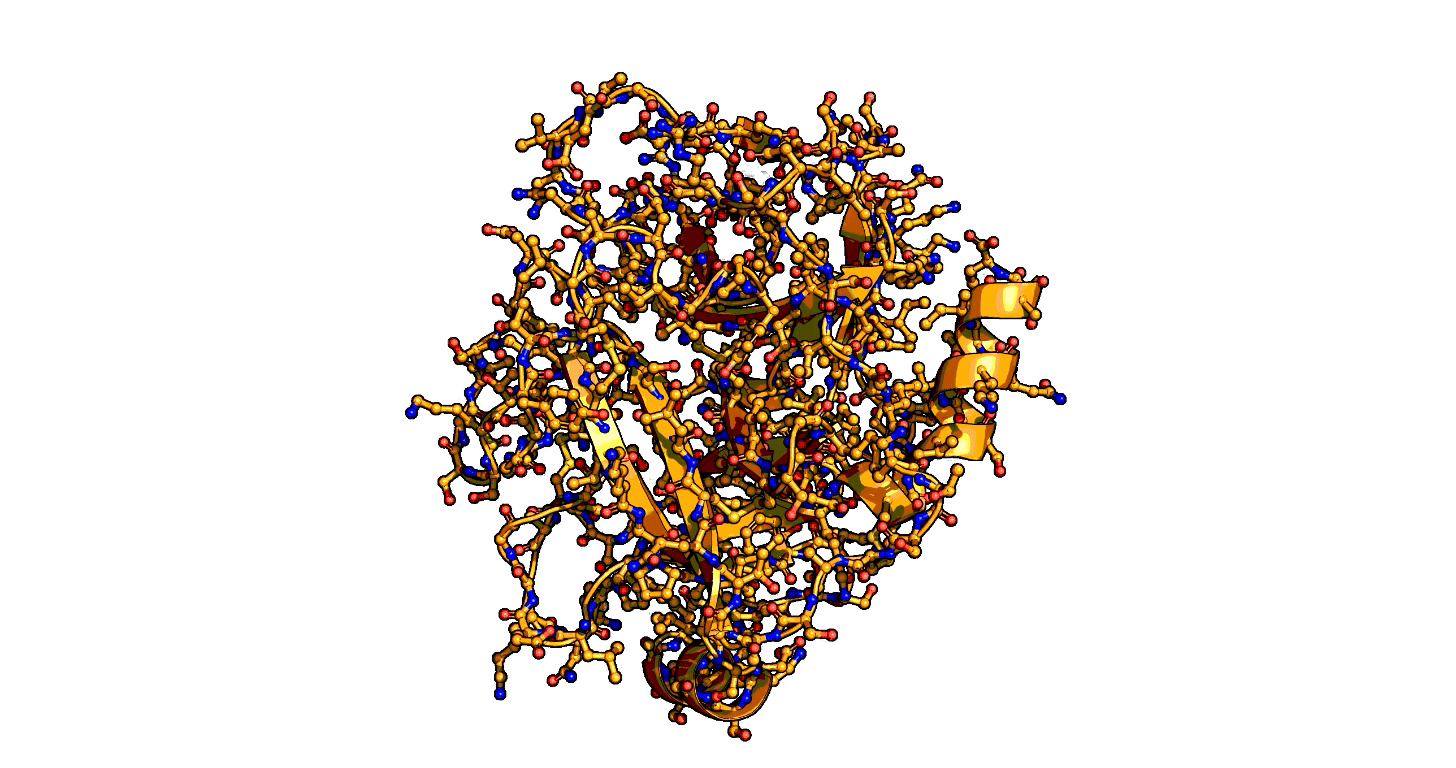
Animation showing the transition state of a serine protease – an example of how enzymes are always in motion. | Siyuan Du
Elusive entities
Until 1926, biochemists were perplexed by reactions that were exclusive to living systems, chalking them up to a mysterious “vital force.” That year, James Sumner isolated the first known enzyme, urease, in a Nobel Prize-winning study. Biochemists have since spent the last century trying to understand how enzymes make reactions so fast and so specific. That is, they have described how they work in words, not quantitatively, and that has led to debate and contrasting views on how they work.
Du and Herchlag built upon a widely held view among biophysicists that enzymes are not a single structure. Instead, they focus on what they term “ensembles,” showing how enzymes move between different physical states – or conformational ensembles – during catalysis.
“All existing models use some degree of positioning of the reacting chemicals and groups of chemicals on the enzyme that aid reactions,” Herschlag explained. But debate has raged about how much positioning matters, with no way to measure it without this new ‘ensemble’ approach Herschlag and Du have taken.
“Enzymes are constantly in motion – in an ensemble of states – and the rate of the reaction is determined by the probabilities within the ensemble,” Du elaborated.
As their subject, the authors chose a family of enzymes known as the serine proteases, which is the family most biochemistry textbooks use to explain enzymatic processes to budding biochemists.
Exploring these ensembles and comparing reaction states on enzymes to states of uncatalyzed enzymes in pure water, Herschlag and Du broke down enzyme catalysis to the individual energetic contributions at the precise place where enzyme and target molecule meet during a reaction, known as the active site, to understand how they work chemically and physically to speed up reactions.
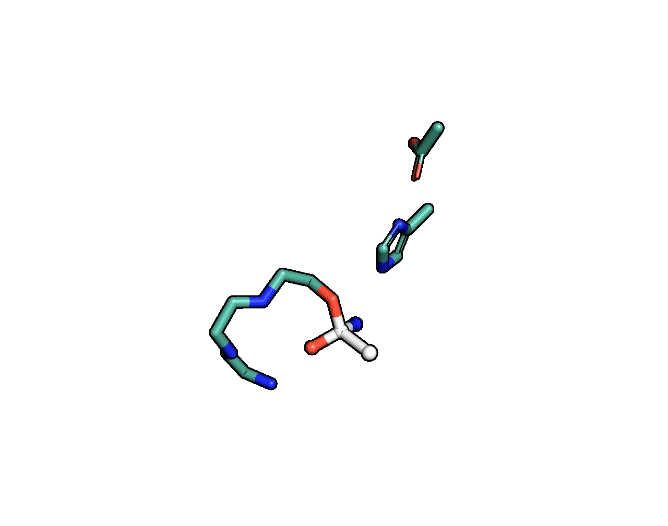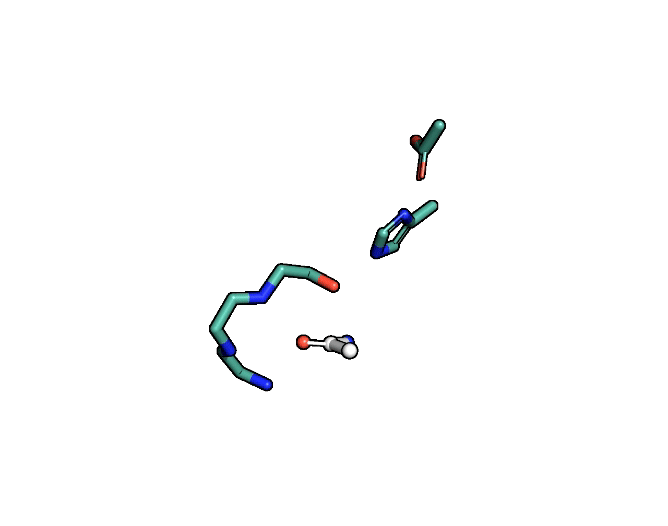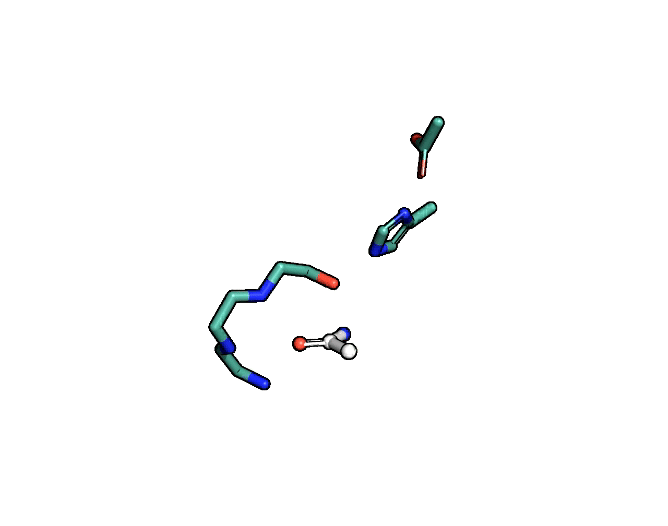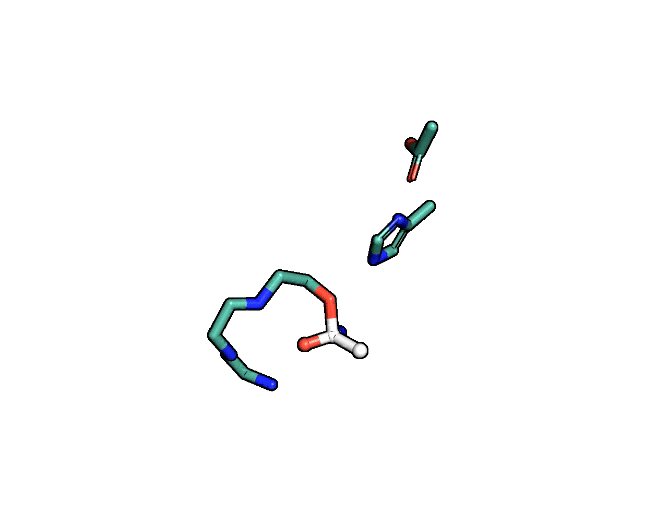
A close-up showing the serine protease reaction at the active site, place where enzyme and target molecule meet during a reaction. | Siyuan Du
Potential energy
In one example, Du noted how an enzyme’s oxygen atom at the active site encroaches on a carbon atom on the molecule it is “attacking.” It is a little like a coiled spring Du said, but cautioned against a too-literal interpretation, noting that the ways individual atoms and groups of atoms move differ from the smooth motion of a spring.
“There’s a little bit of tension forcing these atoms together and, when the reaction happens, all that pent-up energy pushes the reaction forward and it leads to a much faster reaction,” she said.
Du then noted that these catalytic strategies appeared across all the serine proteases, as expected, but also in more than 100 other enzymes.
“Nature has evolved these mechanisms independently in multiple enzyme families – this is not an isolated feature, but catalytic mechanisms that have been discovered multiple times by nature through evolution. This means we may be able to copy nature and use this and other features to design and build new enzymes,” Du said.
As for what comes next, Herschlag and Du said that the ability to explain the extraordinary abilities of these important biochemicals in simple chemical terms could revolutionize how biochemistry is taught and could speed new science in a number of important fields.
“Bottom line,” Herschlag said, “We need to better understand enzymes before we can expect to have real power over them and engineer better ones.”
For more information
Additional Stanford authors include graduate Rachael Kretsch, former undergraduate trainee Jacob Parres-Gold, former postdoctoral scholar Elisa Pieri, former postdoctoral scholar Vinícius Wilian Cruzeiro at the SLAC National Accelerator and Stanford, graduate student Mingning Zhu at SLAC and Stanford, former graduate student and postdoctoral scholar Margaux Pinney, former postdoctoral scholar Filip Yabukarski, former postdoctoral scholar Jason Schwans, and Todd Martínez, the David Mulvane Ehrsam and Edward Curtis Franklin Professor of Chemistry and professor of photon science at SLAC.
Herschlag is also a member of Stanford Bio-X and the Stanford Cancer Institute, and a faculty fellow of Sarafan ChEM-H. Martínez is also a member of Bio-X and a principal investigator at the Stanford PULSE Institute.
This research was funded by the National Science Foundation.
Media contacts
Taylor Kubota, University Communications: tkubota@stanford.edu