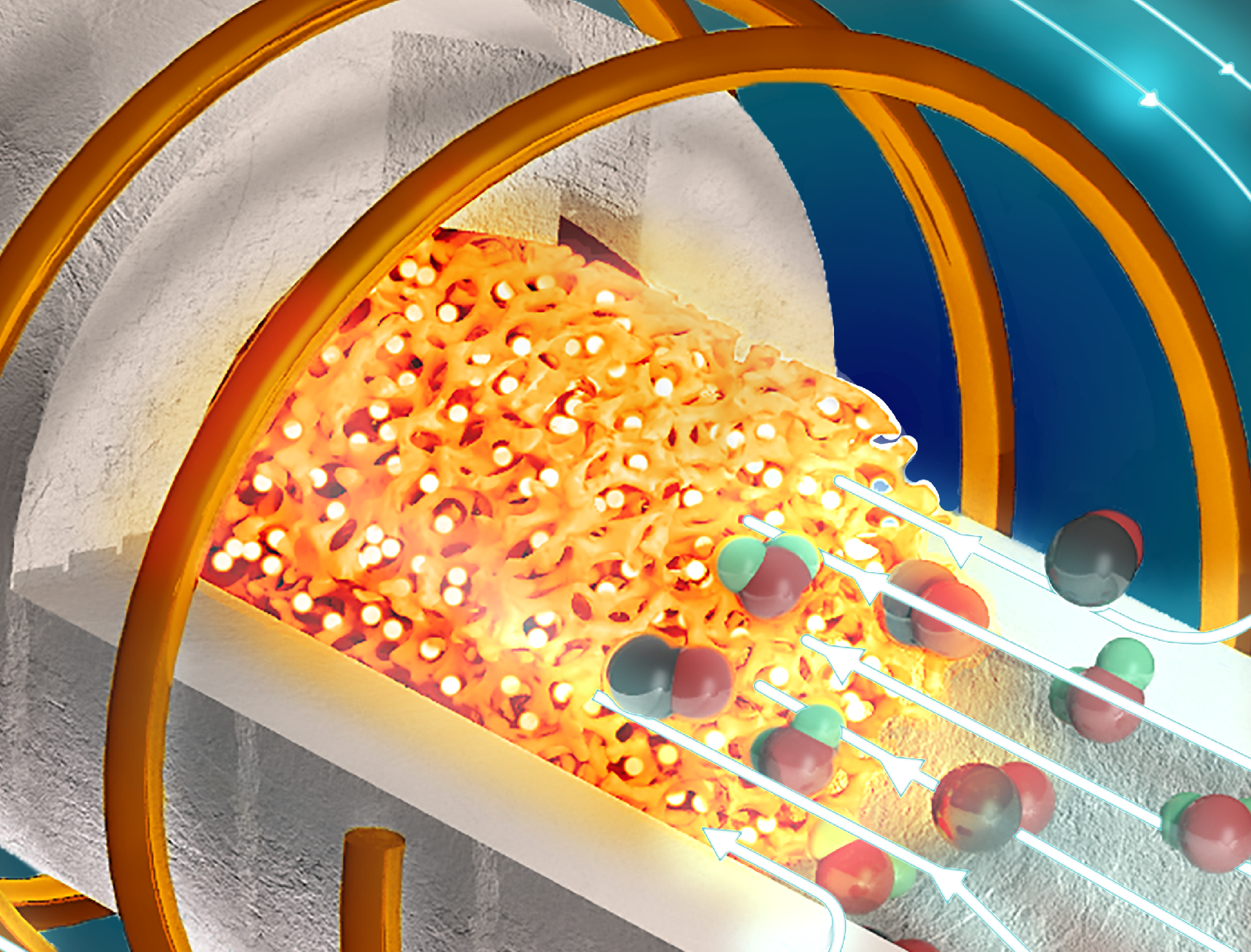
This image depicts the inductively heated metamaterial reactor with catalysts filling the ceramic foam baffle. It is producing carbon monoxide and water from the reverse water gas shift reaction. | Dolly Mantle
Currently, industrial processes in the U.S. account for approximately a third of the country’s carbon dioxide emissions – even more than the annual emissions from passenger vehicles, trucks, and airplanes combined. Decarbonizing this sector is a challenging but vital step in mitigating impacts on our future climate.
Researchers at Stanford Engineering have designed and demonstrated a new type of thermochemical reactor that is capable of generating the immense amounts of heat required for many industrial processes using electricity instead of burning fossil fuels. The design, published Aug. 19 in Joule, is also smaller, cheaper, and more efficient than existing fossil fuel technology.
“We have an electrified and scalable reactor infrastructure for thermochemical processes that features ideal heating and heat-transfer properties,” said Jonathan Fan, an associate professor of electrical engineering at Stanford and senior author on the paper. “Essentially, we’re pushing reactor performance to its physical limits, and we’re using green electricity to power it.”
Heating with induction
Most standard thermochemical reactors work by burning fossil fuels to heat a fluid, which then flows into pipes in the reactor – like a boiler sending hot water to cast iron radiators in an old house, but with better insulation and at much higher temperatures. This requires a fairly large amount of infrastructure and there are many opportunities to lose heat along the way.
The new electrified reactor uses magnetic induction to generate heat – the same sort of process used in induction stoves. Instead of having to transport heat through pipes, induction heating creates heat internally within the reactor, by taking advantage of interactions between electric currents and magnetic fields. If you wanted to inductively heat up a steel rod, for example, you could wrap a wire around it and run an alternating current through the coil. These currents create an oscillating magnetic field which, in turn, induces a current in the steel. And because steel is not a perfect conductor of electricity, some of that current turns into heat. This method effectively heats the whole piece of steel at the same time, rather than creating heat from the outside in.
Adapting induction heating for the chemicals industry is not as easy as just turning up the heat. Industrial reactors need to evenly create and distribute heat in a three-dimensional space and be much more efficient than the average stovetop. The researchers determined that they could maximize their efficiency by using particularly high frequency currents, which alternate very quickly, in conjunction with reactor materials that are particularly bad conductors of electricity.
The researchers used new, high-efficiency electronics developed by Juan Rivas-Davila, an associate professor of electrical engineering and co-author on the paper, to produce the currents they required. They then used those currents to inductively heat a three-dimensional lattice made of a poorly conducting ceramic material in the core of their reactor. The lattice structure is just as important as the material itself, Fan said, because the lattice voids artificially lower the electrical conductivity even further. And those voids can be filled with catalysts – the materials that need to be heated to initiate chemical reactions. This makes for even more efficient heat transfer and means the electrified reactor can be much smaller than traditional fossil fuel reactors.
“You’re heating a large surface area structure that is right next to the catalyst, so the heat you’re generating gets to the catalyst very quickly to drive the chemical reactions,” Fan said. “Plus, it’s simplifying everything. You’re not transferring heat from somewhere else and losing some along the way, you don’t have any pipes going in and out of the reactor – you can fully insulate it. This is ideal from an energy management and cost point of view.”
Electrified industry
The researchers used the reactor to power a chemical reaction, called the reverse water gas shift reaction, using a new sustainable catalyst developed by Matthew Kanan, a professor of chemistry in the School of Humanities and Sciences and co-author of the paper. The reaction, which requires high heat, can turn captured carbon dioxide into a valuable gas that can be used to create sustainable fuels. In the proof-of-concept demonstration, the reactor was over 85% efficient, indicating that it converted almost all electrical energy into usable heat. The reactor also demonstrated ideal conditions for facilitating the chemical reaction – carbon dioxide was converted to usable gas at the theoretically predicted rate, which is often not the case with new reactor designs.
“As we make these reactors even larger or operate them at even higher temperatures, they just get more efficient,” Fan said. “That’s the story of electrification – we’re not just trying to replace what we have, we’re creating even better performance.”
Fan, Rivas-Davila, Kanan, and their colleagues are already working to scale up their new reactor technology and expand its potential applications. They are adapting the same ideas to design reactors for capturing carbon dioxide and for manufacturing cement, and they are working with industrial partners in the oil and gas industries to understand what those companies would need to adopt this technology. They are also conducting economic analyses to understand what system-wide sustainable solutions would look like and how they could be made more affordable.
“Electrification affords us the opportunity to reinvent infrastructure, breaking through existing bottlenecks and shrinking and simplifying these types of reactors, in addition to decarbonizing them,” Fan said. “Industrial decarbonization is going to require new, systems-level approaches, and I think we’re just getting started.”
For more information
Fan is a member of Stanford Bio-X and the Wu Tsai Neurosciences Institute. Rivas is a member of the Stanford Cardiovascular Institute and the Wu Tsai Neurosciences Institute. Kanan is a member of Stanford Bio-X and the Maternal & Child Health Research Institute.
Additional Stanford co-authors of this research include visiting scholar Pinak Mohapatra; postdoctoral researcher Chenghao Wan; and graduate students Calvin H. Lin, Zhennan Ru, Connor Cremers, Dolly L. Mantle, Kesha Tamakuwala, and Ariana Höfelmann.
This work was funded by the Stanford Doerr School of Sustainability Accelerator, the National Science Foundation, the Gates Millennium Scholarship, and the Stanford Graduate Fellowship.
Media contact
Jill Wu, School of Engineering: jillwu@stanford.edu