A team led by chemists at Stanford University has unraveled a longstanding mystery that brings them one step closer to a cleaner, more energy-efficient way to make methanol, an important industrial chemical used in products such as paints, plastics and glues.

Stanford Professor Edward Solomon and graduate student Benjamin Snyder have published new findings on zeolites, ordinary crystalline materials that can transform methane into methanol without added heat or pressure. (Image credit: L.A. Cicero)
For decades, scientists have known that certain zeolites – an ordinary crystalline material – could convert methane into methanol at room temperature, as opposed to the high temperatures and pressures needed for the current process. The question was, how do zeolites work?
“Zeolites are inexpensive and used in everything from catalysts to cat litter,” said graduate student Benjamin Snyder, co-lead author of a study published in Nature about the findings. “In the 1990s, scientists showed that certain iron-containing zeolites have an outstanding ability to convert methane into methanol at room temperature. Our study finally explains how that chemical transformation occurs.”
The Stanford team collaborated on the study with scientists at the University of Leuven (KU Leuven) in Belgium.
The promise of zeolites
Zeolites consist primarily of aluminum, silicon and oxygen. Their porous molecular structure makes them ideal for trapping pet odors and unwanted particles, like radioactive waste. Zeolites are also used as catalysts to make gasoline, diesel fuel and other petrochemical products.
In the 1990s, Russian scientists conducted a series of experiments on synthetic zeolites made with iron. They discovered that iron zeolites exposed to methane gas immediately start cranking out methanol, even at room temperature.
“Iron zeolites are promising catalysts for low-temperature methane conversion,” said study co-author Edward Solomon, a professor of chemistry at Stanford and of photon science at SLAC National Accelerator Laboratory. “But despite nearly three decades of research, it’s unclear how they work.
“Finding an efficient catalytic process for converting methane into methanol could have far-reaching economic implications.”
Active sites
A big challenge for scientists is locating the active site on iron zeolite crystals where catalysis occurs.
“The main difficulty is figuring out how to differentiate an active site from an inactive ‘spectator’ site on the crystal,” Snyder said. “Both contain iron, and that makes it very difficult to distinguish one from the other using conventional methods.”
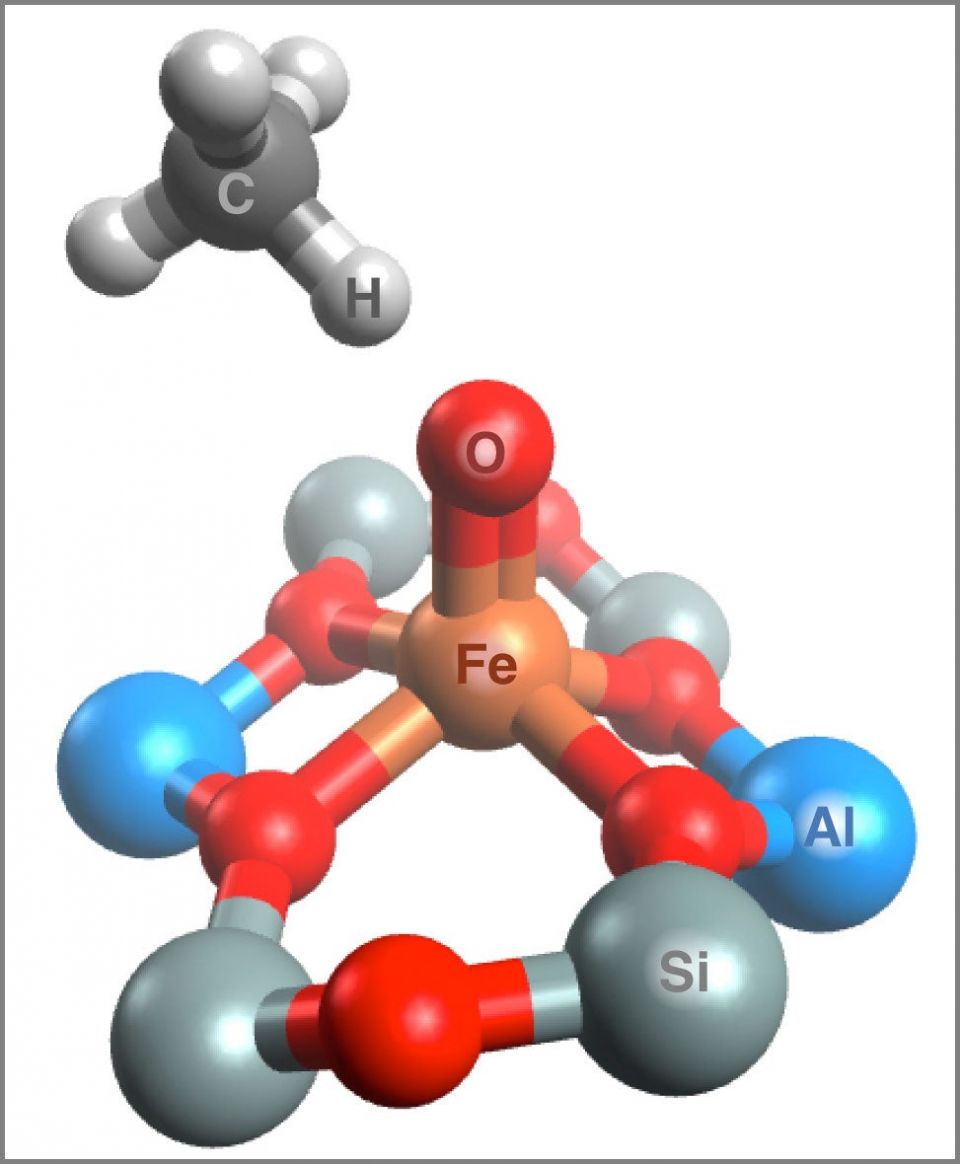
A small methane molecule (upper left) approaches the active site of an iron-containing zeolite. (Image credit: Benjamin Snyder)
The Stanford team applied a series of advanced spectroscopy techniques developed by Solomon’s group to study iron-containing proteins from plants and microbes. That approach allowed them to pinpoint the active site in the zeolites and create detailed computational models of its structure.
“We were then able to show what makes the active site so reactive,” Snyder said. “We found that the iron core of the active site is locked in an unusual, constrained geometry by the zeolite crystal, and this leads to exceptional reactivity with methane.”
Understanding the relationship between catalyst structure and reactivity is a crucial first step in developing environmentally friendly catalysts at scale, he added, but there are many technical hurdles to overcome. For example, most of the methanol made at room temperature gets trapped inside the porous zeolite molecule and has to be removed with water.
“Other researchers can take the design principles we define in our study and run with them,” Snyder said. “Perhaps one day we’ll be able to convert natural gas to methanol right at the point of extraction, without having to transport the gas to industrial-scale plants that require a massive input of energy. That technology may be many years off, but our findings represent an important step forward.”
Former Stanford postdoctoral researcher Pieter Vanelderen, now at KU Leuven, is co-lead author of the study. Other authors are Lars Böttger of Stanford, and Max Bols, Simon Hallaert, Liviu Ungur, Kristine Pierloot, Robert Schoonheydt and Bert Sels of KU Leuven.
Funding was provided by the U.S. National Science Foundation and the Research Foundation–Flanders in Belgium.
Media Contacts
Mark Shwartz, Precourt Institute for Energy: (650) 723-9296, mshwartz@stanford.edu
Edward Solomon, Chemistry/Photon Science: (650) 723-9104, Edward.Solomon@stanford.edu
Benjamin Snyder, Chemistry: (203) 313-2839, bsnyder2@stanford.edu