Stanford researchers have for the first time captured the freezing of water, molecule-by-molecule, into a strange, dense form called ice VII (“ice seven”), found naturally in otherworldly environments, such as when icy planetary bodies collide.

At the Linac Coherent Light Source, Stanford scientists used the world’s most powerful X-ray laser to create an extraterrestrial form of ice. (Image credit: Brad Plummer)
In addition to helping scientists better understand those remote worlds, the findings – published online July 11 in Physical Review Letters – could reveal how water and other substances undergo transitions from liquids to solids. Learning to manipulate those transitions might open the way someday to engineering materials with exotic new properties.
“These experiments with water are the first of their kind, allowing us to witness a fundamental disorder-to-order transition in one of the most abundant molecules in the universe,” said study lead author Arianna Gleason, a postdoctoral fellow at Los Alamos National Laboratory and a visiting scientist in the Extreme Environments Laboratory of Stanford’s School of Earth, Energy & Environmental Sciences.
Scientists have long studied how materials undergo phase changes between gas, liquid and solid states. Phase changes can happen rapidly, however, and on the tiny scale of mere atoms. Previous research has struggled to capture the moment-to-moment action of phase transitions, and instead worked backward from stable solids in piecing together the molecular steps taken by predecessor liquids.
“There have been a tremendous number of studies on ice because everyone wants to understand its behavior,” said study senior author Wendy Mao, an associate professor of geological sciences and a Stanford Institute for Materials and Energy Sciences (SIMES) principal investigator. “What our new study demonstrates, and which hasn’t been done before, is the ability to see the ice structure form in real time.”
Catching ice in the act
Those timescales became achievable thanks to the Linac Coherent Light Source, the world’s most powerful X-ray laser located at the nearby SLAC National Accelerator Laboratory. There, the science team beamed an intense, green-colored laser at a small target containing a sample of liquid water. The laser instantly vaporized layers of diamond on one side of the target, generating a rocket-like force that compressed the water to pressures exceeding 50,000 times that of Earth’s atmosphere at sea level.
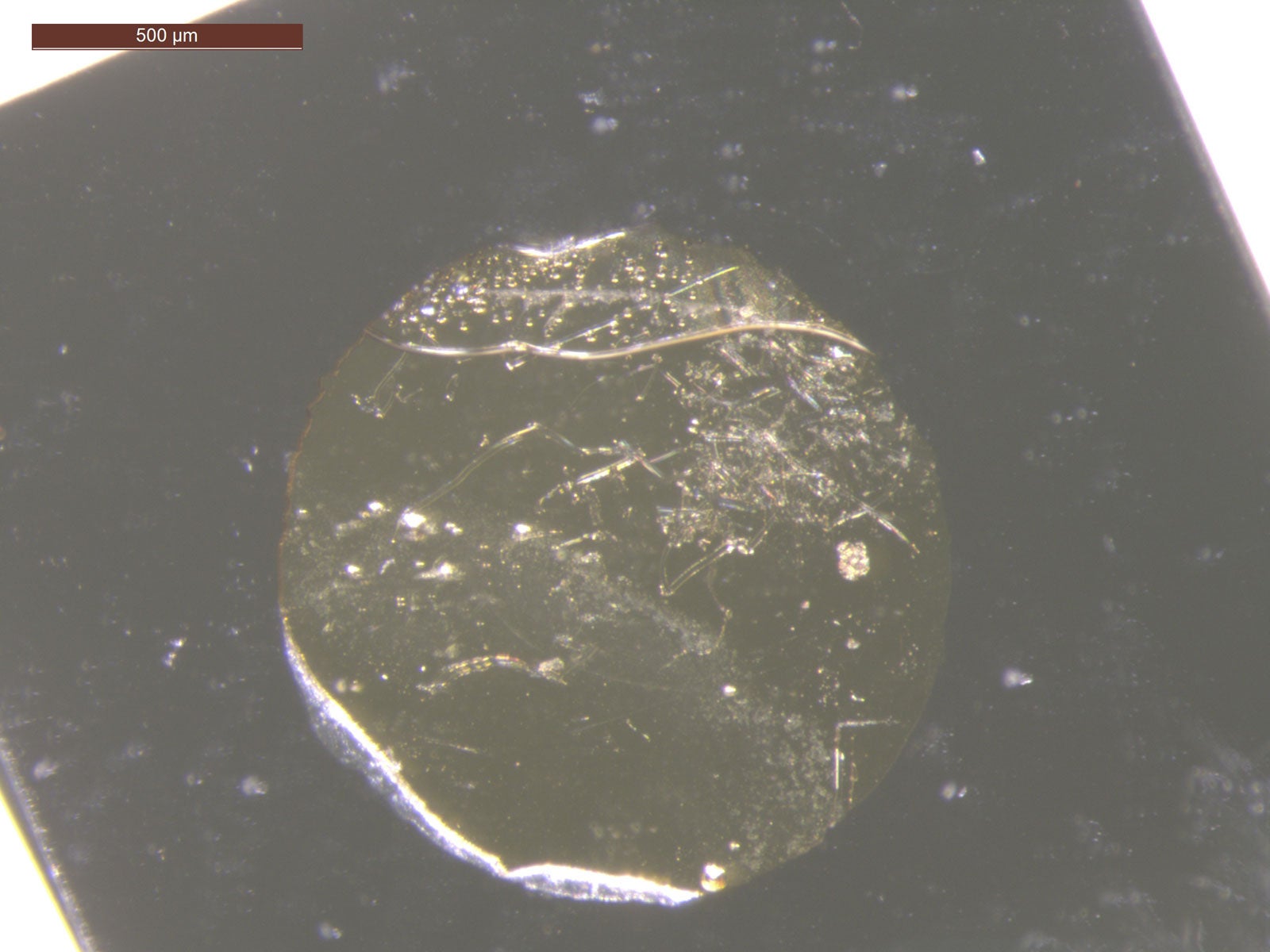
This photo shows a circular water layer sandwiched between a diamond platelet coated with gold and a quartz platelet. The water layer transforms into ice VII after being blasted by an intense green laser. (Image credit: Courtesy Arianna Gleason)
As the water compacted, a separate beam from an instrument called the X-ray Free Electron Laser arrived in a series of bright pulses only a femtosecond, or a quadrillionth of a second, long. Akin to camera flashes, this strobing X-ray laser snapped a set of images revealing the progression of molecular changes, flip book–style, while the pressurized water crystallized into ice VII. The phase change took just 6 billionths of a second, or nanoseconds. Surprisingly, during this process, the water molecules bonded into rod shapes, and not spheres as theory predicted.
The platform developed for this study – combining high pressure with snapshot images – could help researchers probe the myriad ways water freezes, depending on pressure and temperature. Under the conditions on our planet’s surface, water crystallizes in only one way, dubbed ice Ih (“ice one-H”) or simply “hexagonal ice,” whether in glaciers or ice cube trays in the freezer.
Delving into extraterrestrial ice types, including ice VII, will help scientists model such remote environments as comet impacts, the internal structures of potentially life-supporting, water-filled moons like Jupiter’s Europa, and the dynamics of jumbo, rocky, oceanic exoplanets called super-Earths.
“Any icy satellite or planetary interior is intimately connected to the object’s surface,” Gleason said. “Learning about these icy interiors will help us understand how the worlds in our solar system formed and how at least one of them, so far as we know, came to have all the necessary characteristics for life.”
Other co-authors on the study include Cindy Bolme of Los Alamos National Laboratory; Eric Galtier, Hae Ja Lee and Eduardo Granados of the SLAC National Accelerator Laboratory; Dan Dolan, Chris Seagle and Tom Ao of Sandia National Laboratories; and Suzanne Ali, Amy Lazicki, Damian Swift and Peter Celliers of Lawrence Livermore National Laboratory.
Funding was provided by the National Science Foundation, the Los Alamos National Laboratory, the U.S. Department of Energy Office of Science, Fusion Energy Science and the SLAC National Accelerator Laboratory.
Media Contacts
Wendy Mao, School of Earth, Energy & Environmental Sciences: (650) 723-3718, wmao@stanford.edu
Arianna Gleason, School of Earth, Energy & Environmental Sciences: ariannag@stanford.edu
Ker Than, School of Earth, Energy & Environmental Sciences: (650) 723-9820, kerthan@stanford.edu