|
May 26, 2011
Stanford researchers use fluorescent nanotubes to illuminate the inner workings of laboratory mice
Stanford researchers have developed a way to see deeper – and more clearly – into the internal organs of laboratory mice, an improvement useful in the development of new medicines. Fluorescent carbon nanotubes, injected into the mice, provide the clearer images. By Louis Bergeron
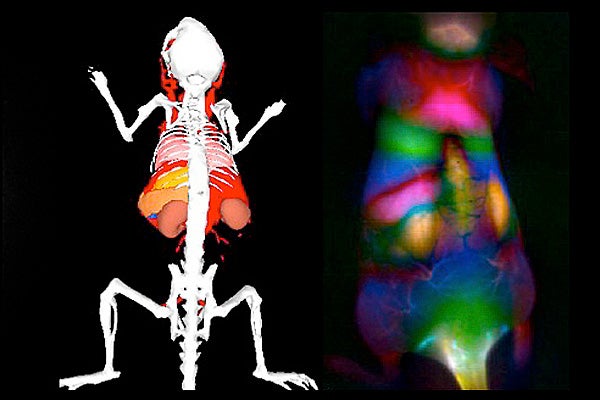
An enhanced color image of fluorescence from single-walled carbon nanotubes (right) shows internal organs of a mouse next to a reference illustration (left). In the fluorescent image, on the left side of the mouse, the pancreas (thin green strip) is sandwiched between a kidney (yellow) and the spleen (pink). In the reference image, the kidneys are orange-brown, the spleen is pumpkin-colored and the pancreas is barely visible as a tiny red triangle between the other two organs. (Image reproduced with permission from PNAS) Developing drugs to combat or cure human disease often involves a phase of testing with mice, so being able to peer clearly into a living mouse's innards has real value.
But with the fluorescent dyes currently used to image the interior of laboratory mice, the view becomes murky a few millimeters under the skin.
Now Stanford researchers have developed an improved imaging method using fluorescent carbon nanotubes that create color images centimeters beneath the skin with far more clarity than conventional dyes provide. For a creature the size of a mouse, a few centimeters makes a great difference.
"We have already used similar carbon nanotubes to deliver drugs to treat cancer in laboratory testing in mice, but you would like to know where your delivery went, right?" said Hongjie Dai, a professor of chemistry. "With the fluorescent nanotubes, we can do drug delivery and imaging simultaneously – in real time – to evaluate the accuracy of a drug in hitting its target."
Researchers inject the single-walled carbon nanotubes into a mouse and then watch as the tubes are delivered to internal organs by the bloodstream.
The nanotubes fluoresce brightly in response to the light of a laser directed at the mouse, while a camera attuned to the nanotubes' near-infrared wavelengths records the images.
By attaching the nanotubes to a medication, researchers can see how the drug is progressing through the mouse's body.
Dai is one of the authors of a paper describing the research published online this month in Proceedings of the National Academy of Sciences.
The key to the nanotubes' usefulness is that they shine in a different portion of the near-infrared spectrum than most dyes.
Biological tissues – whether mouse or human – naturally fluoresce at wavelengths below 900 nanometers, which is in the same range as the available biocompatible organic fluorescent dyes. That results in undesirable background fluorescence, which muddles the images when dyes are used.
But the nanotubes used by Dai's group fluoresce at wavelengths between 1,000 and 1,400 nanometers. At those wavelengths there is barely any natural tissue fluorescence, so background "noise" is minimal.
The nanotubes usefulness is further boosted because tissue scatters less light in the longer wavelength region of the near-infrared, reducing image smearing as light moves or travels through the body.
"The nanotubes fluoresce naturally, but they emit in a very oddball region," Dai said. "There are not many things – living or inert – that emit in this region, which is why it has not been explored very much for biological imaging."
By selecting single-walled carbon nanotubes with different diameters and other properties, Dai and his team can fine-tune the wavelength at which the nanotubes fluoresce.
The nanotubes can be seen immediately upon injection into the bloodstream of mice.
Dai and graduate students Sarah Sherlock and Kevin Welsher, who are also coauthors of the PNAS paper, observed the fluorescent nanotubes passing through the lungs and kidneys within seconds after injection. The spleen and liver lit up a few seconds later.
The group also did some post-production work on digital video footage of the circulating nanotubes to further enhance the image quality, using a process called "principal component analysis."
"In the raw imaging, the spleen, pancreas and kidney might appear as one generalized signal," Sherlock said. "But this process picks up the subtleties in signal variation and resolves what at first appears to be one signal into the distinct organs."
"You can really see things that are deep inside or blocked by other organs such as the pancreas," Dai said.
There are other imaging methods that can produce deep tissue images, such as magnetic resonance imaging (MRI) and computer tomography (CT) scans. But fluorescence imaging is widely used in research and requires simpler machinery.
Dai said that the fluorescent nanotubes are not capable of reaching the depth of CT or MRI scans, but nanotubes are a step forward in broadening the potential uses of fluorescence as an imaging system beyond the surface and near-surface.
Since nanotube fluorescence was discovered about 10 years ago, researchers have tried to make the fluorescence brighter, Dai said. Still, he has been a little surprised at just how well they now work in animals.
"I did not imagine they could really be used in animals to get deep images like these," he said. "When you look at images like this, you get a sense that the body almost has some transparency to it."
Funding for the research came from the National Institutes of Health.
-30-
|